By Edries Yousaf Hajam. Mentored and edited by Eric Bender.
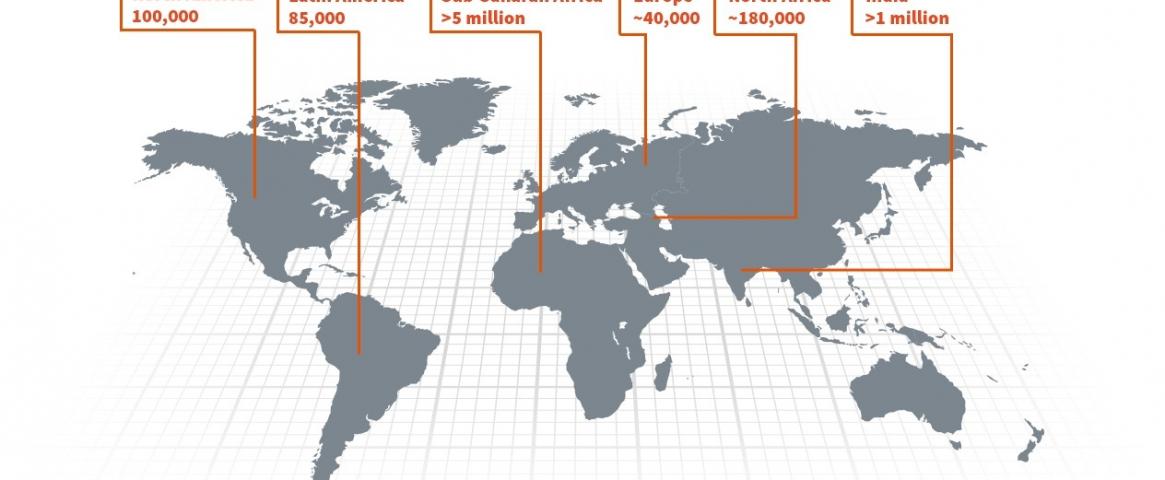
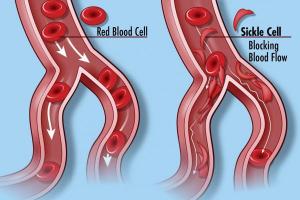
Sickle cell disease (SCD) is a genetic disorder that causes red blood cells to become sickle-shaped instead of disc-shaped like normal cells. Around the globe, at least 300,000 infants are born with SCD each year, mostly in sub-Saharan African and South Asian countries with poor health care systems. People with SCD may suffer from complications such as strokes, lung infections, organ damage, blindness and pain crises requiring frequent hospitalization.
However, a newly developed gene therapy is showing very promising early clinical results for SCD, said researchers at the American Association for the Advancement of Science (AAAS) virtual annual meeting in February.A single dose of gene therapy eventually may prove to restore the health of the red blood cells and to protect patients from developing SCD complications throughout their lifetimes, declared David Williams, chief of hematology and oncology at Boston Children’s Hospital in Boston, Massachusetts.
In healthy humans, red blood cells contain a protein known as hemoglobin, which carries oxygen. Hemoglobin has two forms, one before birth and the other after birth. In some individuals, a small mistake in the gene for the adult protein can make the red blood cells pointy and rigid. These cells then can clump together and fail to deliver oxygen to other cells throughout the body.
Until now, the closest approach to a cure for SCD has been bone marrow transplants, procedures that are very costly and can cause serious side effects, including death.
How do scientists and clinicians plan to correct the genetic mistake? The researchers are developing many strategies, some based on “small molecule” (oral) drugs and others on gene therapies. “It's a great time to be a clinician in the area of sickle cell disease, because for the first time in many years, we have multiple new options,” said Williams.
One main goal is to restart production of the fetal form of the hemoglobin protein, which can function well in adults. This is the route that Williams and his colleagues are taking, by genetically modifying the cells in the bone marrow that generate red blood cells. “Our therapeutic goal is to reverse this switch … to increase the fetal form of the protein and reduce the adult protein,” he said.
This technique uses a patient’s own cells, so a bone-marrow donor is not required and the risk of complications after treatment is reduced.In 2021, preliminary results from a clinical trial that began in 2018 were reported in a New England Journal of Medicine paper. At the AAAS meeting, Williams gave an update on this study, which has enrolled nine participants and followed some of them for more than 40 months. Seven out of the nine patients no longer suffered pain, while one patient experienced mild pain and another had severe pain because of an underlying condition. Most of the patients no longer needed blood transfusions to deal with severe pain crises.
Further trials and many years of follow-up are required to fully assess the therapy’s benefits and risks, Williams said. He and his collaborators are planning a much larger second-phase clinical study.
Additionally, Williams is working with other colleagues to encapsulate the therapeutic material into nanoparticles—very small delivery agents that might make the treatment far easier to administer. “We're investing in the research that would hopefully allow us to adopt this approach to the parts of the world where there are many, many more sickle cell patients than in the United States,” he said. “That's the long-term goal.”
Also at the AAAS session, Gerd Blobel, a professor of pediatrics at the University of Pennsylvania in Philadelphia, discussed various ways in which oral drugs might increase production of fetal hemoglobin.
Several biological mechanisms can help red blood cells make the switch from fetal to adult protein, Blobel explained, and small-molecule drugs might be able to clamp down on this switch. It also might be possible to combine multiple drugs in a single pill, trying to tip hemoglobin production enough that a third of the proteins are in the fetal form. That would be enough working hemoglobin to offer very helpful therapy for SCD patients, he said.
Jane-Jane Chen, a principal research scientist at Massachusetts Institute of Technology in Cambridge, Massachusetts, wrapped up the session by emphasizing the many significant scientific contributions made in understanding fetal hemoglobin. “It looks quite promising that new and more effective treatments for sickle cell disease are on the horizon in the near future,” she said.
Edries Yousaf Hajam is a graduate student in life sciences at the Institute for Stem Cell Science and Regenerative Medicine (inStem) in Bangalore. He can be reached at edriss.yousuf@gmail.com or @edrissyousuf on Twitter.
Hero image: Sickle cell disease is a genetic condition seen most often among people of African ancestry. The disease is spread around the globe and most prevalent in Africa. (Novartis)