This student story was published as part of the 2023 NASW Perlman Virtual Mentoring Program organized by the NASW Education Committee, providing science journalism practice and experience for undergraduate and graduate students.
Story by Alex Dong
Mentored and edited by Tom Abate
Everyone knows about the power of artificial intelligence because of recent applications like ChatGPT, but researchers have been leveraging the technology in medicine for years. To explain why, Dr. Thomas Fuchs, Dean of Artificial Intelligence and Human Health at Mount Sinai, cites data as the driving factor—tons of it.
“What’s on everybody’s mind these days are the chat applications because they are so impressive,” Fuchs said. To train an AI language model, researchers black out a few words in a sentence and have the model repeatedly predict what words best fill in the blank. “If you do that on humongous scales, the models learn the underlying structure of the data you’re looking at,” Fuchs said. In text, this underlying structure is the framework of human language; in drug discovery, it’s molecular patterns in the natural world.
However, these patterns are incredibly complex, found deep within stores of data generated by medical research—often far too much to be sorted and analyzed by humans. By learning and extracting these patterns, AI models can predict drug properties like toxicity and bioactivity, identify key characteristics to find promising compounds, or even generate new drug molecules from scratch.
These advancements are enabling what’s known as data-driven research. Traditionally, research has been hypothesis-driven, where scientists make informed predictions and test if the data support their hypotheses. “Hypothesis-driven research constrains you drastically based on what you can think of—and we humans are limited with our brain power. While with data-driven research, you have the potential to find things you haven’t thought of before,” Fuchs added.
This was the philosophy that PscyhoGenics, a preclinical research organization focused on central nervous system disorders, used to develop their AI-enabled drug discovery platforms. The company has partnered with Sunovion Pharmaceuticals to develop an antipsychotic known as Ulotaront to treat schizophrenia, currently in Phase 3 clinical trials.
The disease mechanisms of brain disorders like schizophrenia remain a mystery, so PyschoGenics isn’t using the conventional approach for drug discovery. Rather than targeting specific genes associated with the disease, the company is analyzing patterns of behavior—the output of the brain—to screen potential drugs.
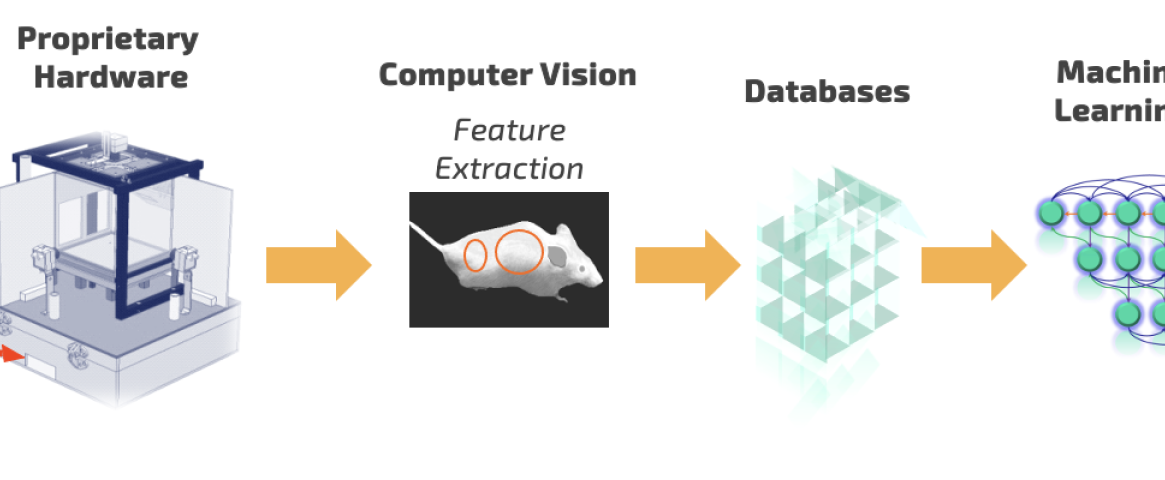
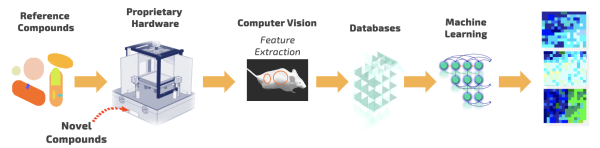 Cartoon workflow for PsychoGenics’ AI-powered SmartCube platform used to discover Ulotaront. Courtesy: PsychoGenics.
Cartoon workflow for PsychoGenics’ AI-powered SmartCube platform used to discover Ulotaront. Courtesy: PsychoGenics.
To effectively measure behavior, PsychoGenics worked with Carnegie Mellon University to develop an AI-enabled platform called SmartCube. Candidate drugs or reference compounds are injected into mice, which are then placed into a controlled environment where their behavior is tracked for about an hour.
“SmartCube is essentially a box that challenges mice in a variety of ways, while multiple cameras give us a 3D picture of everything that mouse is doing,” said Dr. Emer Leahy, neuroscientist and CEO of PsychoGenics. “We’re collecting millions of data points, so we’re using machine learning to extract patterns of behavior and make predictions about therapeutic utility.”
By leveraging AI to analyze behavior in a high-throughput manner, PsychoGenics only had to test around 300 compounds—a stark reduction in time and costs compared to the industry average of 2,500—before Ulotaront emerged as the development candidate to take forward into clinical testing.
Another remarkable feature of Ulotaront is that it avoids a specific “anti-target” known as Dopamine receptor D2. Currently, all antipsychotics on the market target this receptor in one way or another, but these treatments have many side effects—including catalepsy, metabolic syndrome, and weight gain. Moreover, these treatments don’t work in all patients, nor do they address all symptoms.
“Current treatments for schizophrenia typically address the positive symptoms—hallucinations, delusions, hearing of voices—but they don’t address what’s referred to as negative symptoms—apathy, social withdrawal, flat effect—that are very disabling to patients,” Leahy said. Since Ulotaront is an antipsychotic that doesn’t target D2, PyschoGenics hopes that it will have fewer side effects, better manage negative symptoms, and offer improved response rates.
AI tools are facilitating drug discovery, but the compounds themselves are still undergoing the usual regulatory processes. Ulotaront has already demonstrated its safety and tolerability in Phase 1 and 2 clinical trials. However, two recent Phase 3 studies assessing efficacy found that it failed to demonstrate a statistically significant improvement over the placebo, so Sunovion is currently in talks with the FDA on how to proceed.
While the future for Ulotaront is uncertain, this is only the beginning for how advancements in AI are driving a new wave of therapies. The more data that is generated, the more AI models can learn, and the more they will improve. “So, we’re constantly learning, constantly updating algorithms, and improving based on the information that we gather,” Leahy said.
Alex Dong is a senior at Yale University double-majoring in Biomedical Engineering and the Humanities with a certificate in Journalism. He was a former editorial intern at Fast Company, covering health, technology, and leadership. He also serves as Editor-in-Chief of the Yale Scientific Magazine, the nation’s oldest college science publication, and the Yale Layer, his campus’ mental health publication. Follow him on LinkedIn or email him at alex.dong@yale.edu.
The NASW Perlman Virtual Mentoring program is named for longtime science writer and past NASW President David Perlman. Dave, who died in 2020 at the age of 101 only three years after his retirement from the San Francisco Chronicle, was a mentor to countless members of the science writing community and always made time for kind and supportive words, especially for early career writers. Contact NASW Education Committee Co-Chairs Czerne Reid and Ashley Yeager and Perlman Program Coordinator Courtney Gorman at mentor@nasw.org. Thank you to the many NASW member volunteers who spearhead our #SciWriStudent programming year after year.
Founded in 1934 with a mission to fight for the free flow of science news, NASW is an organization of ~ 2,600 professional journalists, authors, editors, producers, public information officers, students and people who write and produce material intended to inform the public about science, health, engineering, and technology. To learn more, visit www.nasw.org