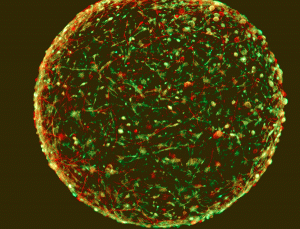
WASHINGTON — Tiny brains made cookie-cutter style could speed discoveries and complement — or some say, replace — mouse models now routinely used in laboratory research on neurological diseases such as Parkinson’s and Alzheimer’s.
The spherical devices, about the size of a fly’s eye, were developed by Dr. Thomas Hartung and his team at the Center for Alternatives to Animal Testing at The Johns Hopkins Bloomberg School of Public Health. Hartung spoke at a Feb. 13 symposium during the American Association for the Advancement of Science (AAAS) annual meeting.
These new mini-brains are the first among a lineup of miniature versions to have nerve cells that, as in humans, are coated with insulating cells called myelin. This is key, because this myelin sheath facilitates transmission of nerve signals, and cells with myelin present a more accurate picture of the way human neurons function. These small organ models, so-called organoids, which can be produced quickly and duplicated exactly, could be used for testing the effects of new drugs or toxins on the brain.
“I see hundreds of thousands of applications beyond what our little group can do,” Hartung said. “And that’s why we want to make them easily available.”
The group is establishing a company called ORGANOME to develop and ship mini-brains to labs across the country. Hartung’s mini-brain system is still in its early stages, however, and its effectiveness for various applications still needs to be proven.
“I’m selling hopes,” he said. “I hope they’re substantiated.”
Fast access to the devices could allow researchers to obtain crucial preliminary results more rapidly, or to test hypotheses quickly using an identical mini-brain as a perfectly matched control. In addition, the mini-brains can be tailored by using stem cells generated from adult skin cells — so-called pluripotent stem cells — from different people.
“One of the advantages of this technique is in terms of its efficiency and its effectiveness in producing large numbers of cell cultures,” said Dr. Wilson Compton, deputy director of the National Institute on Drug Abuse, who also spoke at the symposium. “That’s quite a remarkable achievement.”
Hartung contrasted his mini-brains with more complex brain models he called the Ferraris of the organoid world.
“We are not the first, and we are not the most fancy of these human brains,” Hartung said, “Our goal was to produce Mini Coopers. Produce them fast — but all of them identical.”
Other, more sophisticated mini-brains produced in recent years include work by Dr. Madeline Lancaster of the Medical Research Council in the U.K. Those models have the advantage of being larger, with more complex and differentiated structures, as in human brains. But they can’t be produced quickly, cannot be replicated identically, and are prone to damage.
“Those are the fantastic brain models we have … The problem is, each of them is different. And they are growing so slowly that the cells in the middle are starting to rot, because you don’t have a vasculature,” Hartung said.
But what makes the mini-brains so desirable is the very thing that limits their applicability: They are simple structures — just tangles of nerve cells with spontaneous electrical activity, not differentiated into brain regions. But many areas of brain research, such as those on conditions such as depression and addiction, frequently require analysis of interaction between different brain regions, not just between individual nerve cells. As such, animal models are not likely to go out of use anytime soon.
“Sometimes the complexity of the neurobiology mandates the use of the whole circuits,” said Dr. Wayne Drevets, mood disease area leader at Janssen Pharmaceuticals, who also spoke at the symposium.
The new mini-brains show some complexity in terms of more cells types — four — than previously seen, including some called oligodendrocytes, that form the myelin sheath that surrounds and insulate neurons, increasing their signaling speed.
“Forty percent of our axons are myelinated, which has never been described in vitro,” Hartung said.
But the mini-brains have many hurdles to scale before they could be put into general use. For example, government regulatory processes that look at toxicity and effects of chemical compounds still require animal models.
“We will have to go through a validation process to show that our models are more predictive or as predictive to force the change,” Hartung said.
Nicole Wetsman is a senior neuroscience major at Bowdoin College in Brunswick, Maine. She is the editor-in-chief of the Bowdoin Orient, where she’s served as a writer and editor for three years. Her work has also appeared at WNPR, The Beaker and the (Connecticut) Valley Press. Follow her on Twitter @NicoleWetsman.