By Daniel Erenstein
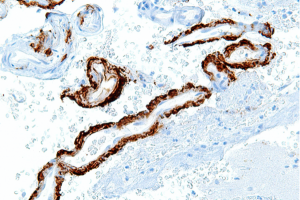
Fragments of amyloid precursor protein aggregate in β-amyloid plaques, seen here in dark brown. These plaques have been found in the brains of patients with Alzheimer’s disease. (Wikimedia Commons.)
But far less is known about the interactions of these bacteria and their products with other parts of the body, and these interactions could hold a particularly important role in human health.
“We’ve always thought of the mouth as somehow in isolation, that oral health does not somehow impact the rest of the body,” said Purnima Kumar, DDS, Ph.D., professor of periodontology at Ohio State University, during a Feb. 8 session at the American Association for the Advancement of Science annual meeting.
Scientists, though, are increasingly looking to the oral microbiome for answers to questions about health and disease.
“The time is absolutely right for us to start putting the mouth back into the body,” Kumar said during the session “Killer Smile: The Link Between the Oral Microbiome and Systemic Disease.”
Panelists highlighted three systemic diseases — diabetes mellitus, rheumatoid arthritis, and Alzheimer’s disease — and their unexpected connection to disturbances in the oral microbiome. A common thread running through all three diseases is an association with periodontitis, gum disease triggered by the accumulation of bacteria, viruses, and even fungi in dental biofilm, or plaque, on the surface of teeth.
In health, there is peace between oral microbes and our immune system. Healthy and frequent communication — molecular diplomacy between bacteria and immune surveillance — maintains stable relations. But microbial imbalances due to bacterial buildup in plaque can cause inflammatory immune reactions, resulting in the gradual breakdown of the barrier between biofilm and gum tissue.
“When you have gum disease, that crosstalk, that communication, that harmony is broken down,” Kumar said.
When bacteria subsequently invade our gum tissues, there are consequences for human disease.
Mark Ryder, DMD, professor of periodontology at University of California, San Francisco, studies the role of one such bacterium, Porphyromonas gingivalis, in disease. This bacterium secretes enzymes called gingipains, which are essential for its survival.
In the case of Alzheimer’s disease, these gingipains can travel through the bloodstream, cross the blood brain barrier, and accumulate in brain regions like the hippocampus, which is involved in memory. There, they help break down amyloid precursor protein into fragments, which group together in deposits found in Alzheimer’s disease.
Further study of gingipains and other microbial products could provide insight into a “critical early event in the initiation and progression of Alzheimer’s disease,” Ryder said.
Similarly, rheumatoid arthritis can be triggered by immune responses to other by-products of P. gingivalis, including protein antibodies that cause joint inflammation, according to Iain Chapple, BDS, Ph.D., professor of periodontology at the University of Birmingham.
Past research on links between the oral microbiome and systemic disease has even shown that these effects can travel a two-way street.
This is apparent in the research of Dana Graves, DDS, DMSc, professor of periodontics at University of Pennsylvania, whose work has examined the effects of diabetes on our microbiome, and vice versa.
“Diabetes impacts the mouth in a very profound way,” said Graves, adding that the inflammatory responses to bacteria caused by diabetes lead to disruption of the microbiome.
“This bidirectionality is something we saw first with diabetes, we’ve seen it now with rheumatoid arthritis, and it appears now that we’re starting to see it with chronic kidney disease,” Chapple said. “We need to start really digging down into [biological mechanisms] to understand more about that relationship.”
However, the verdict is still out on how much bacterial products such as gingipains contribute to disease. For Ryder and others, the existing data is insufficient — fully answering that question depends on carefully constructed clinical trials.
“When we’re trying to establish a link between something like the microbiome and the mouth and Alzheimer’s, association studies are important, the actual underlying biological mechanisms are important, but finally what sort of seals the deal of course is the actual effects of intervention,” Ryder said.
An ongoing clinical trial with more than 600 patients is evaluating the success of gingipain inhibitors in preventing symptoms of Alzheimer’s disease. The results, expected by the end of this year, could carry implications not just for the treatment of Alzheimer’s disease but any disease with underlying roots in the oral microbiome.
Regardless of the results, it’s clear that breaking out the toothbrush and floss every day is crucial to our overall well-being.
Daniel Erenstein is a senior at UC Davis studying neurobiology, physiology, and behavior, with a minor in professional writing. He is editor-in-chief of The Aggie Transcript, an undergraduate life sciences journal at UC Davis. As a B3 (Calisi) Lab researcher, they study changes to the amygdala of single-parent offspring and contribute to workshop development and outreach for the Sci Comm Faculty Training Program at UC Davis under the mentorship of Dr. Alexandra Colón-Rodríguez. You can find him on Twitter @scicommdaniel or email him at derenstein15@gmail.com.
This story was edited by NASW member Karl Leif Bates, who served as Erenstein's mentor during the NASW-AAAS Spring Virtual Mentoring Program.
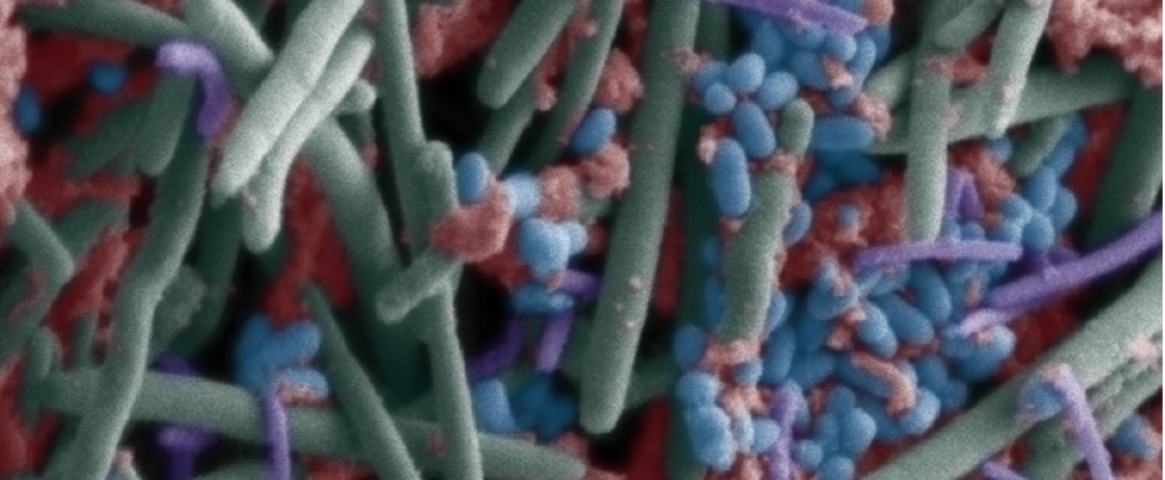
Hero image: The oral microbiome as seen through a scanning electron microscope. (Wikimedia Commons.)