By Emily Driehaus. Mentored and edited by Jenessa Duncombe.
As the world continues to face the threat of climate change, clean water is one resource that could end up in short supply due to extreme weather events like droughts and major storms.
Desalination of seawater has been proposed as a solution, but desalination methods are both financially taxing and energy-intensive, so they are not widely used. However, new physics-driven approaches may offer a more energy-efficient and cost-effective method for water purification processes like seawater desalination as the planet faces a worsening water crisis.
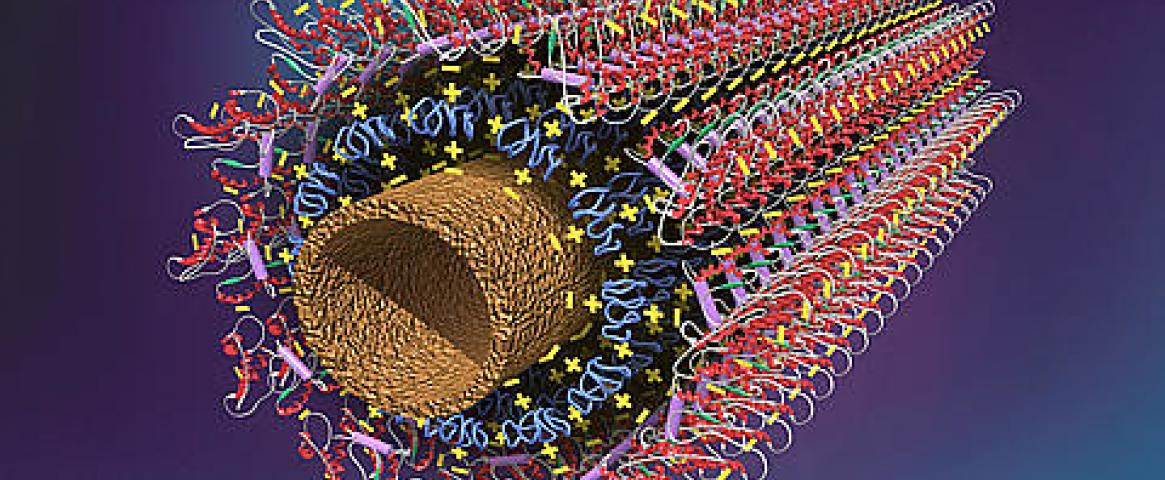
Researchers presented some of these techniques at the annual meeting of the American Association for the Advancement of Science in February during a session dedicated to understanding the physics of clean water. One approach presented by Zuzanna Siwy, a researcher at the University of California, Irvine, and the Center for Enhanced Nanofluidic Transport at the Massachusetts Institute of Technology, involves using precision nanopores to allow for select ions to pass through a channel while preventing others from going through.
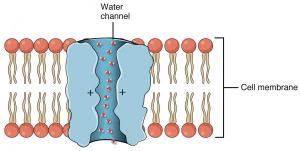
“The research is very much inspired by biological channels in the cell membrane, which transport ions and molecules with an amazing efficiency, as well as selectivity,” Siwy said.Certain types of cells in our bodies, such as kidney cells, have channels called aquaporins, which allow water to pass in and out of cells quickly and efficiently. Aquaporins only allow water through, making them a design model for synthetic nanopores made to selectively filter ions in water.
“Aquaporins fulfill a very important role in the kidneys, specifically they work as desalination units,” Siwy said. “They allow water to pass through but they do not allow any other ions to pass through, including protons.”
While aquaporins were thought to be the most efficient pore transport system for water, nanotechnology research has brought new discoveries about how water behaves in these tiny tubes. Researchers found that 0.8-nanometer carbon nanotubes embedded in a lipid membrane performed better than an aquaporin. The nanotube is so small that it prevents other ions from passing through while allowing water to flow through the tube.
“It transported more efficiently than aquaporins, something which before was considered impossible,” Siwy said. “The fact that the nanotube is only 0.8 nanometers in diameter also induces ion selectivity.”
While nanostructure research can provide more insight into methods for ion separation, it can also lead to a greater understanding of physical phenomena, such as the behavior of water in carbon nanotubes.
“We hope … we will be able to discover new physics, which will be ultimately useful for water purification and also interesting for scientists in general,” Siwy said.
The World Health Organization estimates that 785 million people lack access to basic drinking water today and that half the world’s population will be living in a water-stressed area by 2025. Using synthetic nanopores as a seawater desalination technique could help alleviate impacts in areas most susceptible to water shortages.
While precision nanopore technology is still far from being used on a large scale, Siwy said using nanopores as model purification systems can inform design principles and can be used to fine-tune systems based on ion selectivity and pore surface interactions.
Emily Driehaus is a senior at Loyola University Chicago majoring in anthropology and journalism with a minor in environmental communication. She is a former science communication intern at Hydroviv and exhibition development intern at The Field Museum of Natural History. Follow her on Twitter @EmilyDriehaus or email her at emilydriehaus@gmail.com.
Hero image: "Carbon Nanotube" by Pacific Northwest National Laboratory CC BY-NC-SA 2.0